Calorimetry: Isoperiboles thermal conductivity calorimeter
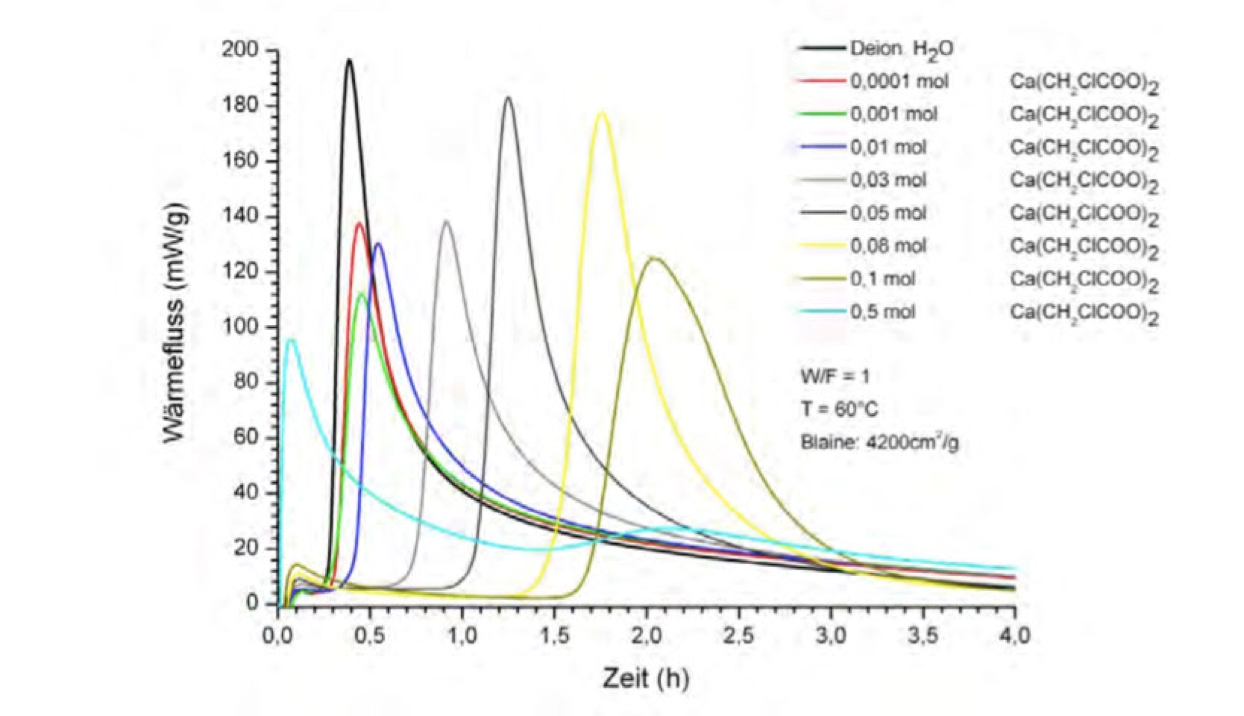
- Quantitative determination of reaction heats
- Hydration reactions as a function of the specific surface area
- Quality control of cement clinkers and cements
- Temperature influences on hydration (isoperibolic mode)
- Special properties of alumina cements
- Investigation and influence of additives and aggregates
- Lime hydration and carbonation reactions
- Polymerisation of glasses
- Carbonation reactions of cements and mortars
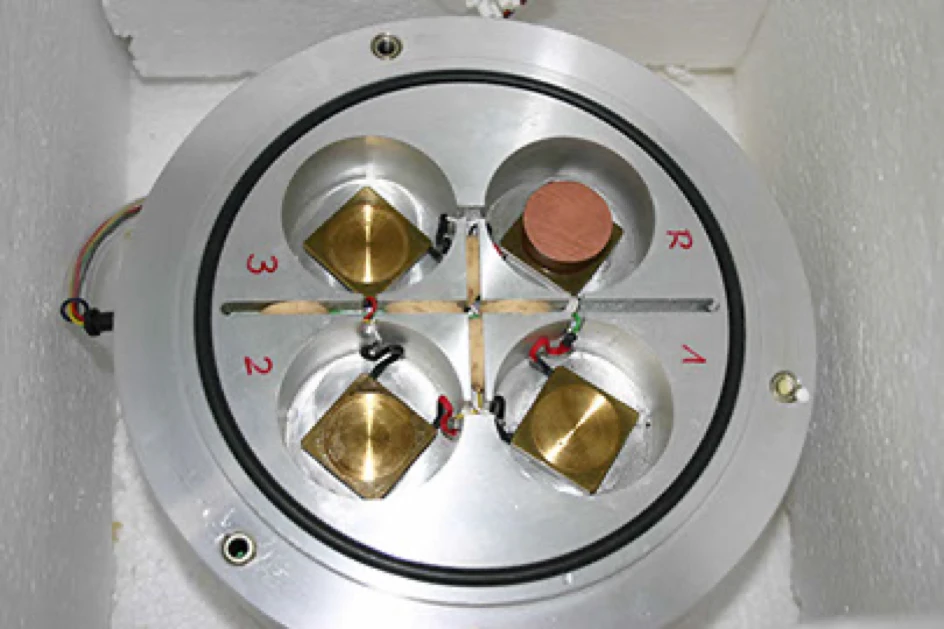
Left: Isoperiboles heat flow calorimeter with hot additive
Right: Calorimeter: Aluminium block with 3 measuring stations and reference
Areas of application
- Quantitative determination of reaction heats
- Hydration reactions as a function of the specific surface area
- Quality control of cement clinkers and cements
- Temperature influences on hydration (isoperibolic mode)
- Special properties of alumina cements
- Investigation and influence of additives and aggregates
- Lime hydration and carbonation reactions
- Polymerisation of glasses
- Carbonation reactions of cements and mortars